FDA Public Advisory Committee Findings About Breast Implants
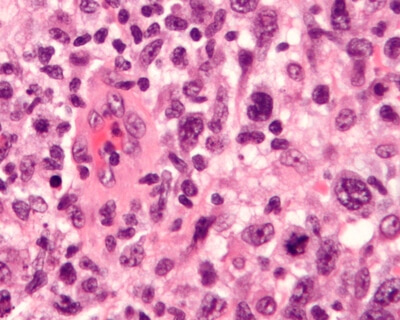
Image of anaplastic large cell lymphoma by Nephron - Own work, CC BY-SA 3.0, Link
THE FDA IS WORKING TO ENSURE THE SAFETY OF SILICONE BREAST IMPLANTS.
On March 25th and 26th, 2019, the Food & Drug Administration (FDA) held a Public Advisory Committee meeting to discuss the risks and benefits of breast implants used in breast augmentation and reconstruction. There were two HOT Topics discussed which included breast implant associated anaplastic large cell lymphoma (BIA-ALCL) and Breast Implant Illness (BII).
What is Breast Implant Associated Anaplastic Large Cell Lymphoma (BIA-ALCL)?
BIA-ALCL is a rare and highly treatable type of lymphoma that develops around textured breast implants. Lymphoma is a cancer of the infection-fighting T-cells of the immune system, not a type of breast cancer. First described in 2007, the BIA-ALCL has been identified in fewer than 500 patients worldwide over the past 12 years. Patients typically present with fluid around the breast implant which can be readily diagnosed with a simple aspiration biopsy of the fluid surrounding the breast implant. Early diagnosis can be achieved for most patients, which can lead to high cure rates with an en-bloc removal of the capsule and breast implant. Removal of breast implants in asymptomatic patients is currently NOT recommended by any health authority, including the FDA.
What Are the Next Steps If I Have a Symptom?
Patients who develop swelling around their breast implants should see their physician to be evaluated with a physical exam and further testing. The treating physician should perform a physical examination of the breasts. Following the physical exam, patients with BIA-ALCL symptoms may receive a magnetic resonance imaging (MRI) and/or an ultrasound of the symptomatic breast. If fluid or a mass is found on the MRI or Ultrasound, a needle biopsy will be used to drain and collect the fluid in the breast This fluid will be tested for BIA-ALCL. This test will confirm diagnosis or rule out BIA-ALCL. Keep in mind this is a rare tumor, therefore, most fluid collections around breast implants are not due to BIA-ALCL.
What is Breast Implant Illness (BII)?
There are a number of women with silicone breast implants who suffer real systemic symptoms. The condition has been referred by some as BII or Breast Implant Illness. Therefore, the question of whether breast implants are the direct cause of these symptoms has been studied by many different groups of medical professionals and data submitted to the FDA. Between 1992 and 2006, the U.S. FDA reviewed a large body of evidence concerning the question of breast implants and any associated disease process. The conclusion at the time, showed no correlation between leaking or intact breast implants and the development of symptoms. Another study in 2011 confirmed these findings. However, we take the symptoms described by patients seriously and will continue to support any ongoing investigation into breast implants and other medical devices.
Additional Information
If you are a patient with questions or concerns about your breast health, your breast implants or BIA-ALCL or BII, please contact our office at 480-970-2580. We are happy to provide you with additional information and are also seeing patients interested in performing screening with MRI for silent rupture or any other breast implant related issues.

Trackbacks