Dysport®: An Overview
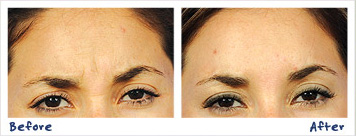
According to the American Society of Plastic Surgeons (ASPS), 6.7 million Botulinum Toxin Type A injections were performed in the US in 2016. However, what the public may not know is that Botulinum Toxin Type A injections are not exclusive to BOTOX®, but also to its competitors. Primarily, Dysport®.
The Botulinum Toxin
The ASPS explains the botulinum toxin as “a purified substance that’s derived from bacteria”. It is used to treat crow’s feet, frown lines, forehead furrows, and skin bands on the neck. It typically costs $385, and is not normally covered by health insurance. The effects are typically visible a few days after the procedure, although patients would need additional treatment in three to six months’ time. The procedure is usually completed in 15 minutes or so.
Over the years, BOTOX® has been the primary choice of doctors when it comes to providing Botulinum Toxin Type A. On April 29, 2009 however, the U.S. Food and Drug Administration (FDA) approved the use of Dysport® for therapeutic and aesthetic uses. It was approved for the “temporary improvement in the appearance of moderate to severe glabellar lines in adults younger than 65 years of age” and “the treatment of cervical dystonia in adults to reduce the severity of abnormal head position and neck pain,” according to Drugs.com.
Dysport® Explained
Dysport®, is classified as abobotulinumtoxinA, “is an acetylcholine release inhibitor and neuromuscular blocking agent for the temporary improvement in the appearance of moderate to severe glabellar lines, upper limb spasticity in adults, and lower limb spasticity in pediatric patients.” It was first registered in the United Kingdom in 1990, and is licensed in over 80 countries with eight different indications. There have also been many clinical trials and studies about the medicine, with over 1,300 peer-reviewed publications.
As is very clear from this article, Dysport® is also used to treat a number of conditions. On August 1, 2016, the FDA further approved it for the treatment of pediatric lower limb spasticity in children above the age of two.
Dysport® as a non-surgical cosmetic treatment.
Similar to BOTOX® Cosmetic, Dysport® works by blocking “nerve activity in the muscles, causing a temporary reduction in muscle activity.” It has the ability to smoothen the face of any imperfections, so that the patient would appear younger and fresher.
We will continue to discuss Dysport® in our upcoming blogs. Stay tuned for more information!
If you are interested in treating your wrinkles of the forehead, crow's feet or frown lines with Dysport® then schedule a personal consultation online or call the office at 480-970-2580.

Trackbacks